There have been a wave of eye drop recollects this yr, with the U.S. Meals and Drug Administration (FDA) not too long ago asserting a nationwide voluntary recall of one other 27 merchandise.
On Oct. 27, the company launched a listing of 26 merchandise that buyers shouldn’t buy and will cease utilizing on account of ”the potential danger of eye infections that might end in partial imaginative and prescient loss or blindness.”
A couple of days later, the FDA added yet another product to the listing.
MORE EYE DROPS RECALLED AFTER FDA WARNING
The merchandise are marketed beneath the next manufacturers, the company acknowledged in its announcement:
- CVS Well being
- Chief (Cardinal Well being)
- Rugby (Cardinal Well being)
- Ceremony Help
- Goal Up & Up
- Velocity Pharma

This yr has seen a wave of eye drop recollects, with the FDA asserting a nationwide voluntary recall of 27 merchandise within the nation. (iStock)
”Ophthalmic drug merchandise pose a possible heightened danger of hurt to customers as a result of medicine utilized to the eyes bypass among the physique’s pure defenses,” the FDA famous.
The company referred to as for the producers to recall all plenty of the desired merchandise on account of ”unsanitary situations within the manufacturing facility and optimistic bacterial check outcomes from environmental sampling of crucial drug manufacturing areas within the facility.”
Customers ought to ”correctly discard” the merchandise, the FDA really helpful.
RITE AID CLOSING 154 STORES IN 15 STATES: HERE’S THE LIST
”The current uptick in recollects has been linked to potential bacterial contamination on account of unsanitary and inferior high quality manufacturing services that didn’t meet the required FDA ‘good practices’ in manufacturing,” Dr. Ronald Benner, president of the American Optometric Affiliation (AOA) in Missouri, instructed Fox Information Digital.
”It’s vital to notice that eye drops are secure when manufactured and used correctly.”
On Oct. 27, the company launched a listing of 26 merchandise that buyers shouldn’t buy and will cease utilizing on account of ”the potential danger of eye infections that might end in partial imaginative and prescient loss or blindness.” (iStock)
On Nov. 3, the FDA introduced that Cardinal Well being Inc. and Harvard Drug Group LLC had issued voluntary recollects for his or her merchandise.
On Nov. 15, Kilitch Healthcare India Restricted adopted swimsuit with its personal voluntary recall.
CVS, Ceremony Help and Goal all stated they might take away the merchandise from their retailer cabinets and web sites, the FDA acknowledged.
CVS PULLS POPULAR COLD MEDICINES FROM STORE SHELVES
”Sufferers who’ve indicators or signs of an eye fixed an infection after utilizing these merchandise ought to speak to their well being care supplier or search medical care instantly,” the FDA stated in its announcement.
Up to now, there have been no experiences of opposed occasions associated to the October recall.
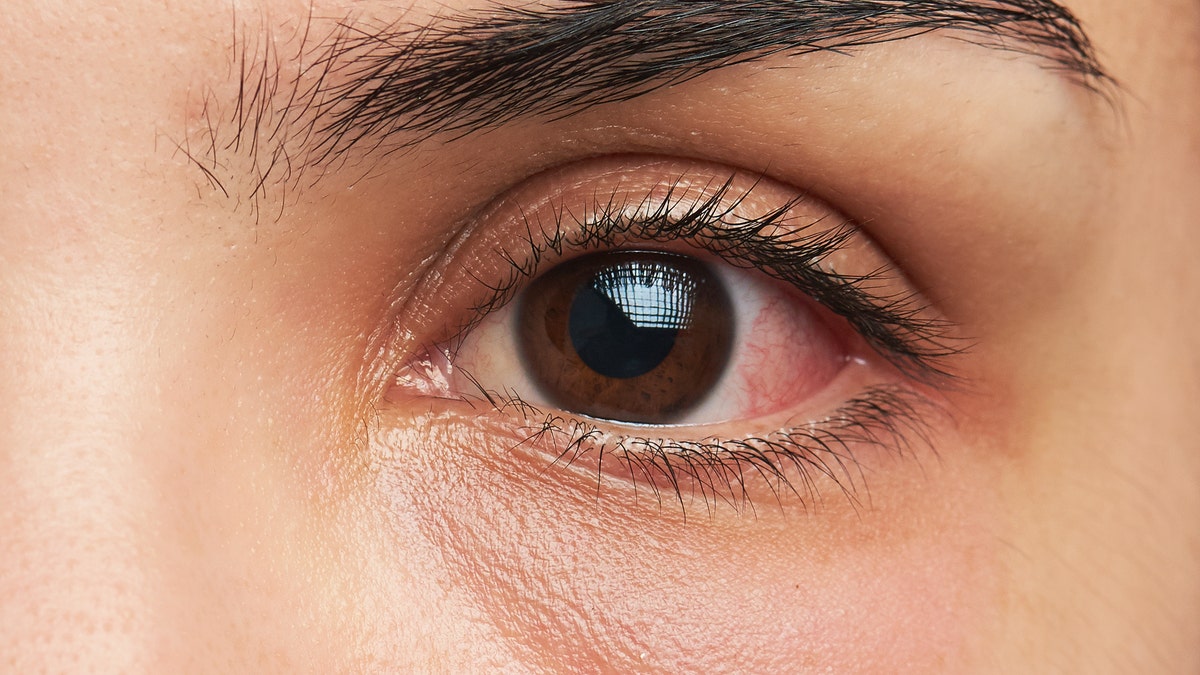
Indicators of a possible eye an infection signs might embody redness of the attention or eyelid, eye ache or irritation, and watery eyes. (iStock)
Earlier within the yr, nonetheless, the January 2023 recall of EzriCare or Delsam Pharma’s Synthetic Tears resulted in 81 folks reporting infections throughout 18 states, Benner famous.
”To this point, 14 folks have gone blind, 4 required enucleation and 4 others died,” he instructed Fox Information Digital.
The FDA can solely make suggestions and doesn’t have the authority to situation obligatory recollects — that’s left as much as the person retailers and producers.
What shoppers ought to do
Utilizing contaminated synthetic tears will increase the danger of eye infections that might end in blindness or demise, Benner warned.
”Sufferers who’ve used these merchandise and/or have indicators or signs of an eye fixed an infection ought to search medical care instantly,” he stated.
FAMILY DOLLAR RECALL OF OTC DRUGS, MEDICAL DEVICES SPANS 23 STATES
”Some eye infections can result in additional issues, particularly if left untreated.”
Indicators of a possible eye an infection signs might embody the next, he stated.
- Redness of the attention or eyelid
- Eye ache or irritation
- Watery eyes
- Feeling of one thing in your eye (overseas physique sensation)
- Elevated sensitivity to gentle
- Blurry imaginative and prescient
Even when somebody has not skilled any of those signs however has used any of the recalled merchandise, Benner stated they need to see their optometrist for a complete examination. (iStock)
Even when somebody has not skilled any of those signs however has used one of many recalled merchandise, Benner stated that individual ought to see their optometrist for a complete examination to evaluate the entrance floor of their eye.
”You probably have used [one of the] merchandise or have skilled any new and/or extended irritation in your eyes, go to AOA.org to ebook an appointment with an AOA physician of optometry close to you,” he really helpful.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Anybody who owns the recalled eye drops ought to comply with the FDA’s pointers for throwing the merchandise away, which can contain taking them to a drug take-back website, Benner famous.
The FDA additionally encourages well being care professionals and sufferers to report opposed occasions or high quality issues with any medication to FDA’s MedWatch Opposed Occasion Reporting program.
Earlier than shoppers deal with themselves with over-the-counter (OTC) eye drops, the AOA recommends consulting with their native optometrist and in addition checking the FDA’s web site for an up-to-date listing of merchandise being recalled.

Anybody who owns the recalled eye drops ought to comply with the FDA’s pointers for throwing the merchandise away, which can contain taking them to a drug take-back website. (iStock)
”When speaking along with your optometrist, it’s vital to share any prescribed and over-the-counter drugs presently in use and ask for his or her skilled opinion,” Benner instructed Fox Information Digital. ”This will additionally assist be certain that an acceptable therapy plan is in place.”
Fox Information Digital reached out to all concerned firms and to the FDA for added remark.
CLICK HERE TO GET THE FOX NEWS APP
CVS Well being offered the next assertion to Fox Information Digital concerning the product recollects: ”Upon receiving notification by the FDA, we instantly stopped the sale in-store and on-line of all merchandise equipped by Velocity Pharma throughout the CVS Well being Model Eye Merchandise portfolio.”
”Clients who bought these merchandise can return them to CVS Pharmacy for a full refund. We’re dedicated to making sure the merchandise we provide are secure, work as supposed and fulfill clients, and are totally cooperating with the FDA on this matter.”
For extra Well being articles, go to www.foxnews.com/well being.

